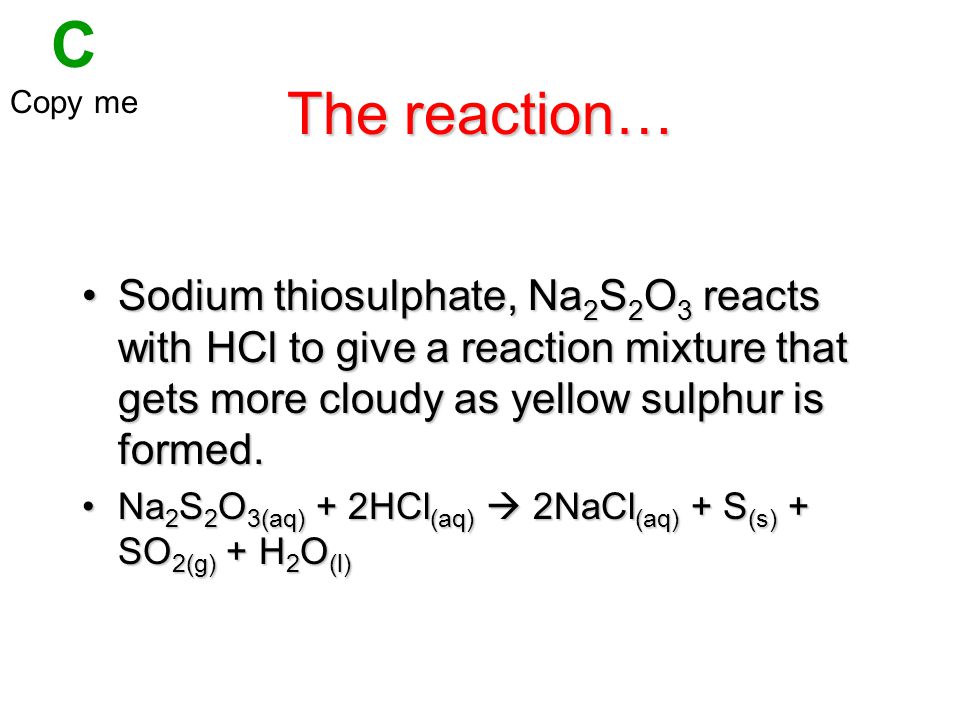
Sodium Thiosulphate and Hydrochloric Acid
Compare the Foaming Capacity of Different Samples of Soap. To set up simple Daniell cell and determine its.
Rate Of Reaction Of Sodium Thiosulfate And Hydrochloric Acid Flinn Scientific
Lab report Introduction.
. Utilization of Spent Hydrochloric acid generated from glass manufacturing industry Hazardous Waste Category Class B15 Inorganic acids Schedule II of HOWM Rules 2016 for manufacturing of Calcium Chloride. Preparation of Organic Compounds. Rate of reaction c Controlled variables.
Aqua regia is a mixture of Concentrated hydrochloric acid 3 parts and concentrated nitric acid 1 part. Dewatered solids from brine treatment. Hydrochloric acid is found naturally in gastric acid.
Determine the enthalpy change during the interaction hydrogen bond formation between acetone and chloroform. Sodium Thiosulphate And Hydrochloric Acid 2. Add 10 ml of hydrochloric acid and 2 g of potassium iodide stopper shake and keep in dark for 15 min.
6 1513 words Rates of reaction between sodium thiosulphate and hydrochloric acid Pages. Dissolve 0125 g of accurately weighed potassium dichromate in 25 ml of water present in a 250 ml erlenmeyer flask. In the production of different types of chlorides and metals.
Drugs Available In Health Facility Refresh Data Filter Data Download PDF. Esterification Reaction between Alcohol and Carboxylic Acid. Sodium thiosulphate reacts with Hydrochloric acid reacts to form a yellow precipitate of sulphur.
Thermochemistry Viva Questions with Answers. Utilization of Flue gas cleaning residue generated from Steel Scrap Melting Induction. As the sodium thiosulphate solution is run in from a burette the colour of the iodine fades.
Oxidation Reactions of Alcohols. Potassium chromate 40 S S Sodium thiosulphate S S Potassium cyanide saturated S S Soybean oil S S Potassium dichromate 40 S S Stannic chloride saturated S S Potassium ferri ferro cyanide S S Stannous. There is a very simple but very effective way of measuring the time taken for a small fixed amount of precipitate to form.
Replenishes depleted thiosulphate stores by acting as sulfur donor necessary for the conversion of CN-O to thiocyanate through the action of sulfur transferase enzyme rhodanese. In the refinement of ore. In addition to removing stains in clothes sodium hypochlorite is also used to clean other types of stains such as mold and tea stains.
Reaction With Aqueous Solutions of Iodine. When added to dil. 3 NaOCl aq 2 NaCl aq NaClO 3 aq To some extent an aqueous solution of sodium hypochlorite is in dynamic equilibrium.
Determine the enthalpy of neutralisation of hydrochloric acid with sodium hydroxide solution. It is a by-product of chlorine manufacture along with sodium hypochlorite and sodium hydroxide. Qualitative Analysis of Carbohydrates.
Waste brines from chlor-alkali plants. Kinetics Study on the Reaction between Sodium Thiosulphate and Hydrochloric Acid. You can find the amount of iodine liberated by titration with sodium thiosulphate solution.
A 2NaOH H 2 SO 4 Na 2 SO 4. It is a common substance found in rocks in all parts of the world and is the main component of shells of marine organisms snails coal balls pearls and eggshells. 134 - Wastes containing sulphides.
And Spent Sodium Thiosulphate. Acetic Acid Glacial 995 Technical Grade 20 litres from 34995 CAD 39995 CAD Sodium Hydroxide NaOH or Caustic Soda Micropearls 2 lbs from 4495 CAD 5995 CAD Sodium Hydroxide NaOH or Caustic Soda Micropearls 25 kgs from 39995 CAD 49995 CAD. Na 2 S 2 O 3 2HCl Dilute Hydrochloric Acid 2 NaCl S SO 2 H 2 O.
Neutralization of hydrochloric acid formed when chlorine gas reacts with water in the airways. Petroleum aqueous refinery condensates. Sodium Thiosulphate is a colorless odorless salt that is inorganic in nature.
Volume and concentration of sodium. If you add dilute hydrochloric acid to sodium thiosulphate solution you get the slow formation of a pale yellow precipitate of sulphur. Typically these solutions contain about 3-8 sodium hypochlorite and 001-005 sodium hydroxide added to reduce decomposition into sodium chlorate and sodium chloride.
In an aqueous solution high temperatures cause it to decompose into sodium chloride and sodium chlorate. P Potassium dichromate Hydrochloric acid Potassium chloride Chromium chloride Water Chlorine. Stand the flask on a piece of paper with a cross.
Silver nitrate solution will give a white ppt. It is a crystalline compound with five molecules of water in it. Calcium carbonate is a chemical compound with the formula CaCO3.
135 - Wastes containing other reactive anions. Hydrochloric acid 30 S S Methylsulfuric acid S S Hydrochloric acid 35 S S Milk S S Hydrocyanic acid S S Mineral oils S U. R Sodium chloride Manganese dioxide Sulphuric acid Sodium hydrogen sulphate Manganese sulphate Water Chlorine.
Hydrogen chloride and ammonia gas. Study of reaction rates of any one of the following. Na 2 S 2 O 3 aq 2HCl aq 2NaCl aq H 2 O l Ss SO 2 g.
Uses for Hydrochloric Acid A highly corrosive mineral acid hydrochloric acid has many industry uses making it known as a workhorse chemical. Hydrochloric acid and no change will be observed when added to dil. 4 1112 words Investigating the kinetics of reaction between sodium thiosulphate and dilute hydrochloric acid Pages.
In solid crystalline pentahydrate form the chemical decomposes at 101C which is also its boiling point. C This is because the temperature. The Rate of Reaction between Hydrochloric Acid and Sodium Thiosulphate Solution Pages.
Sodium Thiosulphate reacts in equimolar amounts stoichiometrically with. Temperature ot sodium thiosulphate solution b Responding variable. Q Sulphur Nitric acid Sulphuric acid Nitrogen dioxide Water.
If you add concentrated hydrochloric acid to a solution containing hexaaquacopperII ions the six water molecules are replaced by four chloride ions. Effect of concentration and temperature on the rate of reaction between Sodium Thiosulphate and Hydrochloric acid. The time taken for the same amount of yellow precipitate to be produced provides a method of measuring the rate of reaction at different concentrations.
Add 100 ml of water to the above mixture and titrate with sodium thiosulphate using starch as the indicator. 1 g of granulated zinc and 20 cm 3 of 02 mol dm 3 hydrochloric acid at 30C b The rate of reaction of set I is higher than that of set II.

Sodium Thiosulfate Hydrochloric Acid Experiment Video Lesson Transcript Study Com

3 Effect Of Concentration On The Rate Of Reaction Between Sodium Download Scientific Diagram

Reaction Of Sodium Thiosulphate And Hydrochloric Acid Ppt Video Online Download

Rate Of Reaction Of Sodium Thiosulfate And Hydrochloric Acid Youtube
Comments
Post a Comment